INTENDEDUSE
The SARS-CoV-2 antigen IVD kit SWAB is an in vitro diagnostic test for the qualitative detection of novel coronavirus antigens in human swab, using the rapid immunochromatographic method. The identification is based on the monoclonal antibodies specific for the novel coronvirus antigen. It will provide information for clinical doctors to prescribe correct medications.
SUMMARY
The novel coronaviruses belong to the β genus.COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The SARS-CoV-2 antigen IVD kit SWAB is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to Novel coroinavirus.
The test strip is composed of the following three parts, namely sample pad, reagent pad and reaction membrane. The reagent membrane contains the colloidal-gold conjugated with the monoclonal antibodies against Novel coroinavirus; the reaction membrane contains the secondary antibodies for Novel coroinavirus, and the polyclonal antibodies against the mouse globulin, which are pre-immobilized on the membrane.
When the test device was inserted into saliva sample,conjugates dried in the reagent pad are dissolved and migrate along with the sample. If Novel coroinavirus is present in the sample, a complex formed between the anti- Novel coroinavirus conjugate and the virus will be caught by the specific anti- Novel coroinavirus monoclonal coated on the T region.
Whether the sample contains the virus or not, the solution continues to migrate to encounter another reagent (an anti-mouse IgG antibody) that binds the remaining conjugates, thereby producing a red line on the region C.
The SARS-CoV-2 antigen IVD kit SWAB product can detect SARS-Cov-2 nucleo-protein(mainly) and spike protein.
More than 90% antibody used in SARS-CoV-2 antigen IVD kit SWAB is anti-nucleoprotein of SARS-Cov-2 and target protein is SARS-Cov-2 nucleoprotein.
The rest antibody used in SARS-CoV-2 antigen IVD kit SWAB is anti-Spike protein and target protein is SARS-Cov-2 Constant fragment of Spike protein.
At present, whether the N501Y in the United Kingdom or the 501Y.V2 in South Africa, the variant fragments are mainly the RBD fragment of the S protein, and the target fragments of the antibodies used inNovel Coronavirus SARS-CoV-2 antigen IVD kit SWAB have not been mutated.So,The SARS-CoV-2 antigen IVD kit SWAB can reliably detect the SARS-Cov-2 variants.
So,The SARS-CoV-2 antigen IVD kit SWAB can reliably detect the nucleo-protein and spike protein of genetic SARS-Cov-2 variants.
REAGENTS
The reagent membrane contains the colloidal-gold conjugated with the monoclonal antibodies against Novel coroinavirus; the reaction membrane contains the secondary antibodies for Novel coroinavirus,and the polyclonal antibodies against the mouse globulin, which are pre- immobilized on the membrane.
DIRECTIONS FOR USE
Allow the test device, specimen, sample extraction buffer to equilibrate to room temperature (15- 30°C) prior to testing. Do not place anything in the mouth including food, drink, gum, tobacco, water and mouth cleaning products for at least 10 minutes prior to collection of oral fluid specimen.
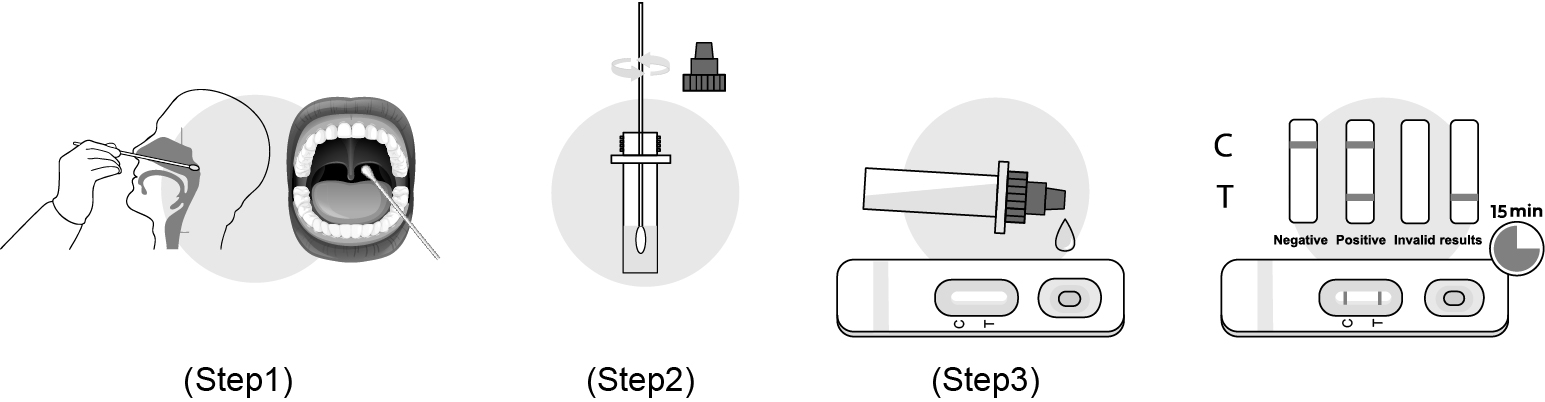
1.Nasopharyngeal swab collection method:The operator holds the swab by the right hand and holds the head of the subject fixedly by left hand. Putting the swab downing backwards the bottom of the nasal cavity and penetrate slowly and gently. Do not overexert to avoid traumatic hemorrhage. When the cusp of the swab touching the paries posterior of the pharyngonasal cavity, letting the swab remain in the place for a few seconds (about 3 seconds) and rotating the swab gently for one cycle, and then remove the swab slowly.
Oropharyngeal swab collection method:The head of the person to be collected is slightly tilted and his mouth is wide open, exposing the pharyngeal tonsils on both sides. Wipe the swab across the root of the tongue. Wipe the pharyngeal tonsils on both sides of the person to be collected back and forth with a little force for at least 3 times, and then wipe up and down the posterior pharyngeal wall for at least 3 times.
2.Immerse the sampled swab into the sample extract to make the sample extract completely penetrate the swab, rotate and squeeze the swab 10 times, take out and discard the swab, close the lid, and shake the extraction tube to make mix the liquid evenly.
3.Transfer 4 drops of mixed sample into the SARS-CoV-2 Test Card vertically, start the timer.
Read the result at 15 minutes. Don’t interpret the result after 20 minutes.
Read the result at 15 minutes. Result after 20 mins will not be valid.
(Please refer to the illustration above)
POSITIVE: Two red lines appear. One red line appears in the control region(C), and one red line in the test region(T). The shade of color may vary, but it should be considered positive whenever there is even a faint line.
NEGATIVE: Only one red line appears in the control region(C), and no line in the test region(T). The negative result indicates that there are no Novel coroinavirus particles in the sample or the number of viral particles is below the detectable range.
INVALID: No red line appears in the control region(C). The test is invalid even if there is a line on test region(T). Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the test procedure and repeat the test using a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.

